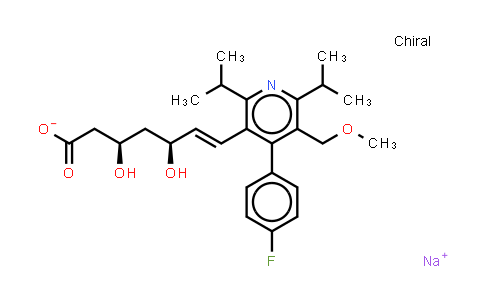
| Chemical Name |
Cerivastatin (sodium) |
| CAS Number |
143201-11-0 |
| MDL Number |
MFCD07787637 |
| Molecular Formula |
C26H33FNNaO5 |
| Molecular Weight |
481.53 |
Introduction of 143201-11-0 :
Cerivastatin sodium is a synthetic lipid-lowering agent and a highly potent, well-tolerated and orally active HMG-CoA reductase inhibitor, with a Ki of 1.3 nM/L. Cerivastatin sodium reduces low-density lipoprotein cholesterol levels. Cerivastatin sodium also inhibits proliferation and invasiveness of MDA-MB-231 cells, mainly by RhoA inhibition, and has anti-cancer effect[1][2]. IC50 & Target: Ki: 1.3 nM/L (HMG-CoA reductase)[1][2][3] In Vitro: Cerivastatin (5-50 ng/mL; 3 days; MDA-MB-231 cells) treatment induces a dose-dependent decrease in cell proliferation of MDA-MB-231 cells (up to 40% inhibition at 25 ng/mL)[1].
Cerivastatin (25 ng/mL; 18-36 hours; MDA-MB-231 cells) treatment induces an arrest of the cell cycle in G 1/S phase after 36 h treatment. This arrest is not observed for a shorter incubation time (18 h)[1].
Cerivastatin (25 ng/mL; 18 hours; MDA-MB-231 cells) treatment induces a marked increase in the level of p21Waf1/Cip1[1].
Cerivastatin (25 ng/mL; 12 hours; MDA-MB-231 cells) treatment increases the p21 transcript in MDA-MB-231 cells[1].
Cerivastatin (10-25 ng/mL; 18 hours) inhibits invasion of MDA-MB-231 cells through Matrigel[1].
Cerivastatin (25 ng/mL; 18-36 hours) delocalizes RhoA and Ras from the membrane to the cytosol and induces morphological changes[1].
Cerivastatin (25 ng/mL; 4-36 hours) induces inactivation of NFκB, in a RhoA inhibition-dependent manner, resulting in a decrease in urokinase and metalloproteinase-9 expression, and concomitantly increases IκB[1]. In Vivo: Cerivastatin is well absorbed, reaching maximal plasma levels in 1-3 hours following oral dosing. In the circulation, Cerivastatin is highly bound to plasma proteins (99.5%), with an elimination half-life of 2-4 hours. Cerivastatin is metabolized predominantly in the liver to three polar metabolites. Two of these metabolites are active, but to a lesser extent compared to parent drug, and the third metabolite is inactive. Plasma concentrations of all metabolites are substantially lower than those of the parent drug. Elimination of metabolites is via the urine (20-25%) and feces (66-73%), while essentially no parent compound is excreted[2].
| Purity |
NLT 98% |
| Storage |
at 20ºC 2 years |
*The above information is for reference only.