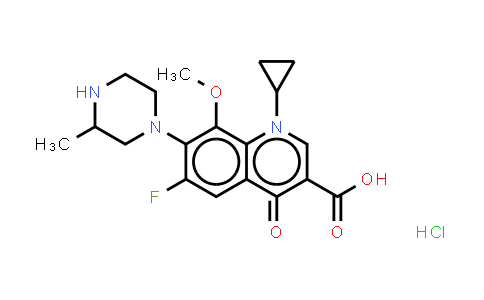
| Chemical Name |
Gatifloxacin (hydrochloride) |
| CAS Number |
121577-32-0 |
| MDL Number |
MFCD09838901 |
| Molecular Formula |
C19H23ClFN3O4 |
| Molecular Weight |
411.86 |
| Synonyms |
AM-1155 (hydrochloride);BMS-206584 (hydrochloride);PD135432 (hydrochloride) |
Introduction of 121577-32-0 :
Gatifloxacin hydrochloride (AM-1155 hydrochloride) is an antibiotic of the fourth-generation fluoroquinolone family, it inhibits the bacterial enzymes DNA gyrase and topoisomerase IV. Target: Antibacterial Gatifloxacin (hydrochloride) is the hydrochloride salt of Gatifloxacin which is an antibiotic of the fourth-generation fluoroquinolone family, that like other members of that family, inhibits the bacterial enzymes DNA gyrase and topoisomerase IV. Gatifloxacin had activity equal to that of tosufloxacin and activity more potent than those of norfloxacin, ofloxacin, ciprofloxacin, and sparfloxacin against the second-step mutants (grlA gyrA; gatifloxacin MIC range, 1.56 to 3.13 microg/ml) and had the most potent activity against the third-step mutants (grlA gyrA grlA; gatifloxacin MIC range, 1.56 to 6.25 microg/ml), suggesting that gatifloxacin possesses the most potent inhibitory activity against singly mutated topo IV and singly mutated DNA gyrase among the quinolones tested [1]. Ophthalmic gatifloxacin 0.3% is at least as effective as ciprofloxacin at healing corneal ulcers infected with Pseudomonas aeruginosa when gatifloxacin is administered less frequently than ciprofloxacin. Trends favored gatifloxacin in fluorescein retention scores [2]. Clinical indications: Bacterial infection FDA Approved Date: Toxicity: Hepatotoxicity; Acute pancreatitis [3]; Torsades de pointes [4]
| Purity |
NLT 98% |
| Storage |
at 20ºC 2 years |
*The above information is for reference only.