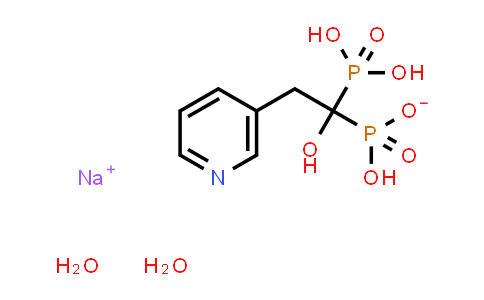
| Chemical Name |
Risedronate (sodium) |
| CAS Number |
115436-72-1 |
| MDL Number |
MFCD09752071 |
| Molecular Formula |
C7H14NNaO9P2 |
| Molecular Weight |
341.12 |
| Synonyms |
Risedronic Acid Sodium |
Introduction of 115436-72-1 :
Risedronate sodium is a pyridinyl biphosphonate which inhibits osteoclast-mediated bone resorption. Target: Risedronate sodium, which was promoted in Croatia a few months ago, is the latest (III) generation of bisphosphonates, the most efficient anti-resorption drugs that inhibit osteoclast-mediated bone resorption and change the bone metabolism. Risedronate sodium is hence the first line of bisphosphonates for the reduction of vertebral and non-vertebral fracture risks in postmenopausal women with osteoporosis or those with a high risk of osteoporosis. It also efficiently prevents bone loss or improves bone density in men and women on a long-term corticosteroid therapy [1]. The administration of 20 and 25 mg/kg risedronate sodium for 4 days led to decreases of parasitemia of 68.9% and 83.6%, respectively. On the seventh day of treatment the inhibitions were 63% and 88.9% with 20 and 25 mg/kg, respectively. After recovering the parasitemia, a dose-response curve was obtained for estimating the ID50 (dose causing 50% inhibition), equivalent to 17 ± 1.8 mg/kg after 7 days of treatment. Four days after the interruption of treatment (11 days postinfection), the parasitemias of the groups treated with 10, 15, 20, and 25 mg/kg/day were 15.3%, 15.9%, 15.2%, and 5.7%, respectively. Conversely, the group that received PBS presented parasitemia of 25.6%. Among the groups treated with risedronate sodium, only the animals that received 25 mg/kg had a significant inhibition of 77.8% (see Table S1 in the supplemental material), demonstrating that even after treatment discontinuation, the parasitemia of the animals remained low in relation to that of the controls [2]. Clinical indications: Bone resorption; Male osteoporosis; Osteogenesis imperfecta; Osteoporosis; Pagets bone disease Toxicity: abdominal pain; anxiety, back pain; belching, bladder irritation; bone disorders and pain; bronchitis; bursitis; cataracts; chest pain; colitis; constipation; depression; diarrhea; difficulty breathing
| Purity |
NLT 98% |
| Storage |
at 20ºC 2 years |
*The above information is for reference only.